Complexing agent/chelating agent/softener
Complexing agents are compounds that can form compounds (called complexes) with metal ions or atoms to mask undesirable chemical properties.
In terms of quantity, the removal of Ca
2+ ions from various media, where precisely these Ca
2+ ions have a disturbing effect, is of greatest importance - this is referred to as softening. Softening therefore means the removal of hardness-forming (Ca
2+, Mg
2+) ions from water or also from dirt.
Complexing agents or chelating agents also remove interfering ions from water or dirt, but in addition to Ca
2+ und Mg
2+ ions, complexing agents also bind (depending on their structure or composition) a whole range of other interfering metal ions such as Fe, Zn, etc.
Definition of water hardness:
1 °dH = 0.18 mmol/l alkaline earth. That is 7.1 mg Ca2+- or 4,3 mg Mg2+-ions per litre of water.
| Hardness range |
Water properties |
°dH |
| 1 |
Soft water |
0 - 7 |
| 2 |
Medium-hard water |
8 - 14 |
| 3 |
Hard water |
15 - 21 |
| 4 |
Very hard water |
> 21 |
Influence of metal ions during the cleaning or washing process.
Effect on surfactants/wetting agents (due to hard water):
Surfactants or wetting agents are impaired in their surface-active effect by the influence and presence of hardness-forming ions. Thus, soaps with calcium ions form poorly soluble lime soaps, which precipitate as residues:

Lime soaps have no surfactant properties and thus extremely reduce the washing performance.
Effect of metal ions in dirt:
Since metal ions strengthen the adhesion of dirt to the surface as well as the cohesion of dirt particles, they should be removed from the respective system if possible. Dirt can be removed much better if metal ions are removed from it.
Formation of gypsum and lime deposits:
With sulphate ions, Ca ions form poorly soluble gypsum:

At temperatures above 60°C, sparingly soluble calcium carbonate (lime) is formed from water-soluble calcium hydrogen carbonate:
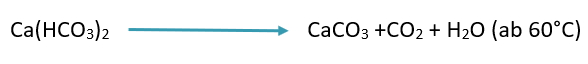
The result in both cases is deposits that are difficult to remove, which damage appliances and greatly impair washing performance.
Decomposition of bleaching agents:
The bleaching effect of various bleaching agents is nullified by the presence of heavy metal ions, as these catalyse the decomposition of bleaching agents.
Mode of action of softeners or complexing agents
The use of complexing agents in washing liquors essentially achieves the following effects:
- Prevention/disintegration of gypsum and lime deposits
- Dispersion of dirt/dirt particles
- Stabilisation of bleaching agents
- Improvement of the swelling capacity of organic dirt
The following three mechanisms of action are distinguished:
- Complexation
- Precipitation
- Ion exchange
Mode of action of various complexing substances and products
Capacities of the various complexing agents
Naturally depends on the type of softener used, the temperature and the respective water hardness:
| Binding capacities of softeners in mg Ca2+-ions per 1 g softener |
| Softener/ Temperature: |
20 °C |
90 °C |
| Phosphonate |
281 |
270 |
| NTA |
204 |
144 |
| Polycarboxylate |
200 |
170 |
| EDTA |
156 |
110 |
| Triphosphate |
113 |
81 |
| Zeolith |
- |
» 81 (bei 60 °C) |
| Citrate |
139 |
21 |
| Gluconate |
90 |
- |
| Soap |
» 65 |
- |
Areas of application of complexing agents in the (chemical) industry
| Surface finishing, electroplating, electrochemistry; electronics industry |
Prevention or reduction of metal/metal salt precipitation in baths.
Prevention of contamination by foreign metal ions in treatment baths.
Improvement of the quality of the end products.
|
| Textile and leather industry |
Stabilisation of peroxide bleaching lyes.
Removal of trace metals to protect dyes.
Reduction of colour tone changes.
Prevention of metal-containing stains.
Prevention of limescale and scale formation.
|
| Pulp and paper industry |
Binding of non-complexed metal ions that catalyse the degradation of hydrogen peroxide and ozone.
To increase shelf life and efficiency of bleaching agents.
To improve the effectiveness of bleaching agents.
|
| Chemical industry |
In a wide variety of industries and manufacturing processes, metal ions have a negative impact on production and the quality of the end products. Complexing agents are used in:
- Polymerisation
- Synthesis of polyesters and polyethers
- Production of inorganic and organic peroxides
- Production of various inorganic products
- Cosmetics industry
- Oil and gas industry
- Production of dyestuffs
- Production of printing inks
- Etc.
|
| Detergent / washing agents |
Water softening.
Dirt trap.
Prevention of gypsum and limescale deposits.
Reinforcement of the cleaning power.
|
Portfolio of complexing agents of Donauchem GmbH
Complexing agents of Donauchem GmbH are traded under the brand name DonPlex. Our portfolio includes products from the product groups of:
- Phosphates/Polyphosphates
- Citric acid/citrates
- Gluconate
- EDTA
- MGDA
- PBTC
- Phosphonic acids/phosphonates
- Polyacrylates
Special products on request.
For further information please contact us for technical and chemical advice.
 www.donauchem.at
www.donauchem.at