Schwefelsäure ist momentan von einer Verknappung betroffen, wie es sie seit dem 2. Weltkrieg nicht mehr gegeben hat. Aktuell gibt es nur beschränkte Mengen an Flüssigschwefel, der als Nebenprodukt bei der Entschwefelung von Rohöl in den Raffinerien anfällt und in Europa hauptsächlich für die Produktion von Schwefelsäure verwendet wird.
Das nehmen wir zum Anlass, um diese Basischemikalie und ihre enorme Bedeutung für die Wirtschaft näher zu beleuchten. Weiters gehen wir auf die Ursachen der schlechten Verfügbarkeit ein und geben eine Einschätzung für die kommenden Monate.
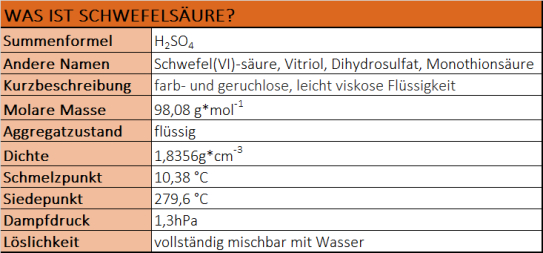
Was ist Schwefelsäure?
Schwefelsäure gehört zu den technisch wichtigsten Chemikalien der Welt und zählt zu den meistproduzierten chemischen Grundstoffen. Der Grund hierfür liegt in den Eigenschaften der vielseitig einsetzbaren Säure.
- Schwefelsäure ist eine farblose, geruchlose und ölige Flüssigkeit.
- Sie ist eine der am stärksten wirkenden Mineralsäuren.
- Schwefelsäure ist in jedem Verhältnis mit Wasser mischbar und gibt dabei hohe Wärmemengen ab.
- In wässrigen Lösungen ist Schwefelsäure eine starke, zweibasige Säure.
- Heiße Schwefelsäure kann Edelmetalle lösen.
- Schwefelsäure zieht Feuchtigkeit aus der Luft an (hygroskopisch).
- In hoher Konzentration wirkt Schwefelsäure wasserentziehend und stark oxidierend.
- Konzentrierte Schwefelsäure wirkt stark ätzend und zerstört organische Stoffe wie Zucker, Baumwolle oder Haut unter Bildung von Kohlenstoff.
Charakterisierung der Schwefelsäure
Welche Herstellungsverfahren gab es im geschichtlichen Verlauf?
Schwefelsäure ist unter der Bezeichnung Vitriol schon seit langer Zeit bekannt. Das Vitriolverfahren ist das älteste Verfahren zur Herstellung von Schwefelsäure. Es beruht auf der thermischen Zersetzung natürlich vorkommender Sulfate, den sogenannten Vitriolen.
Erstmals wurde es in den Schriften des Alchemisten Dschābir ibn Hayyān aus dem 8. Jahrhundert erwähnt und im 13. Jahrhundert von den Alchemisten Albertus Magnus und Basilius Valentinus genauer beschrieben.
Ab dem 16. Jahrhundert wurde aufgrund der steigenden Nachfrage nach Schwefelsäure das Vitriolverfahren im industriellen Maßstab angewendet.
Johann Rudolph Glauber war der Begründer der ersten Schwefelsäure-Manufaktur der Welt, die um 1650 in Nordhausen (Harz) nach diesem Verfahren Schwefelsäure herstellte.
Wegen der nötigen hohen Temperaturen war das Verfahren allerdings recht kostspielig und wurde nach der Entwicklung des Bleikammerverfahrens schnell von diesem verdrängt. Die Schwefelsäure wurde bei dieser Herstellungsmethode durch Verbrennung von Schwefel und Salpeter in Bleibehältern erzeugt.
Nachdem 1793 Nicolas Clément-Désormes und Charles Bernard Désormes entdeckt hatten, dass durch den Einsatz von Luft die Salpetermenge deutlich gesenkt werden kann, konnte das Bleikammerverfahren auch großtechnisch eingesetzt werden.
Moderne Verfahren sind das Kontaktverfahren und das daraus weiterentwickelte Doppelkontaktverfahren.
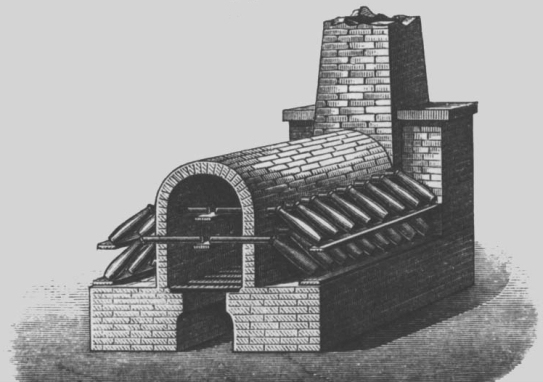
Bild: Galeerenofen, wie er früher für das Vitriolverfahren eingesetzt wurde
Wie wird Schwefelsäure heute hergestellt?
Erdöl und Erdgas sind häufig mit Schwefel verunreinigt. Vor ihrer Verarbeitung werden sie in der Raffinerie entschwefelt. Der hier anfallende flüssige Schwefel kann zur Herstellung von Schwefelsäure genutzt werden. In der großtechnischen Anwendung wird Schwefelsäure heute nach dem sogenannten Doppelkontaktverfahren in einem kontinuierlichen Prozess hergestellt.
In Österreich ist die
Donau Chemie AG der einzige Produzent, der Schwefelsäure für den freien Markt herstellt. In unserer Anlage in Pischelsdorf durchläuft der Herstellungsprozess hochreiner Schwefelsäure insgesamt sechs Schritte:
- Schwefelofen: Verbrennung des flüssigen Schwefels mit Luftsauerstoff zu gasförmigem Schwefeldioxid (SO2). Die dabei erzeugte Abwärme dient zur Dampferzeugung.
- Kontaktturm 1. Kontakt: Oxidation von SO2 (+O2/Sauerstoff gasförmig) mittels Katalysators zu gasförmigem Schwefeltrioxid (SO3). Restanteil an SO2 bleibt, Umsetzung von ca. 85 Prozent des eingesetzten Schwefels. (Kontakt: Katalysatorschüttung zur Umsetzung von SO2 in SO3).
- Zwischenabsorber: Umsetzung des SO3 mit konzentrierter flüssiger Schwefelsäure (H2SO4), bei gleichzeitiger Wasserzugabe.
- Kontaktturm 2. Kontakt: Oxidation des restlichen SO2 (+O2) mittels Katalysators zu SO3. Umsetzung von ca. 99,8 Prozent des eingesetzten Schwefels. (Kontakt: Katalysatorschüttung zur Umsetzung von SO2 in SO3).
- Endabsorber: Umsetzung des restlichen SO3 mit konzentrierter H2SO4 bei gleichzeitiger Wasserzugabe. Die entstehende Abwärme der beiden Absorber wird für die Heißwassererzeugung genutzt.
- Trockenturm: Trocknung der Luft mit konzentrierter H2SO4 für die Schwefelverbrennung und Einstellen der H2SO4 Endkonzentration mit demineralisiertem Wasser auf 96 Prozent.
Darstellung des Doppelkontaktverfahrens
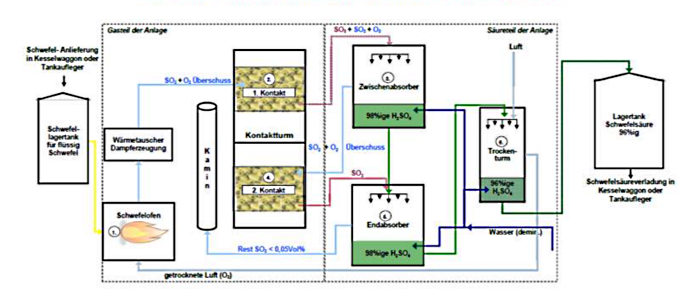
© Donau Chemie AG
Warum ist Schwefelsäure für die chemische Industrie so wichtig und kritisch?
Schwefelsäure wird in ihrer Bedeutung oftmals unterschätzt, obwohl sie weltweit die am meisten produzierte und verwendete Chemikalie ist. Im Jahr 2020 erreichte der globale Schwefelsäuremarkt ein Volumen von fast 284,4 Millionen Tonnen.
Als Schlüsselprodukt der chemischen Industrie wird Schwefelsäure in sehr großen Mengen und in nahezu allen chemischen Prozessen direkt oder indirekt eingesetzt. Insbesondere für Basisprozesse ist sie ein unersetzliches Produkt.
In weiterer Folge bedeutet dies, dass es bei mangelnder Verfügbarkeit nahezu unmöglich oder einfach unrentabel wäre, eine Vielzahl grundsätzlicher und äußerst wichtiger industrieller Verfahren durchzuführen. Viele Produkte können ohne das „Blut der Chemie“ nicht oder nur mit viel größerem Aufwand produziert werden.
Welche Rolle spielt Schwefelsäure für die Wirtschaft?
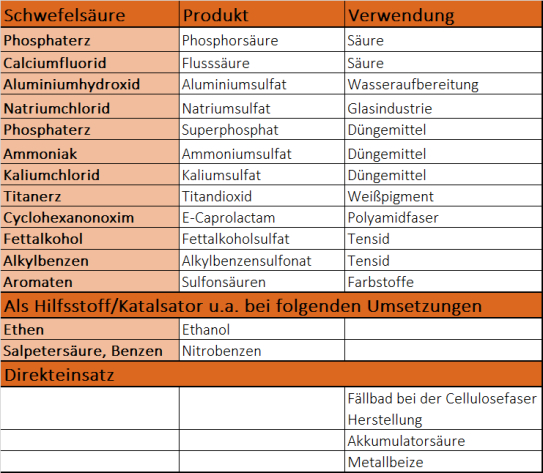
Der größte Mengenanteil von Schwefelsäure kommt in der Produktion von Düngemitteln zum Einsatz, insbesondere zur Herstellung von Superphosphaten, Ammoniumphosphat und Ammoniumsulfat. Auch bei der Produktion von Farbstoffen, Arzneimitteln, Waschmitteln, Kunststoffen oder Sprengstoffen wird sie eingesetzt. Außerdem ist Schwefelsäure für eine Vielzahl organisch-technischer Synthesen als Reaktionspartner, Reaktionshilfsmittel oder Katalysator von großer Bedeutung. Weiters wird sie als Trocknungsmittel oder bei der Verarbeitung von Metallen benötigt.
Die wichtigsten Anwendungen von Schwefelsäure sind:
- Herstellung von Sulfaten (z.B. für Waschmittel, Glas- und Papierindustrie)
- Herstellung von anderen Säuren (z.B. Salzsäure, Salpetersäure, Fluorwasserstoffsäure oder Phosphorsäure)
- Herstellung von Düngemitteln
- Herstellung von Tensiden (z.B. für Wasch- und Reinigungsmittel, Kosmetika, pharmazeutische Produkte)
- Herstellung von Titandioxid (z.B. für Weißpigmente in vielen Farben, Kunststoffen und Lacken)
- Katalysator (z.B. bei Veresterung)
- Reaktionshilfsmittel für organische Synthesen (Nitriersäure)
- Metallbehandlung (z.B. Beizen von Metallen)
- Trockenmittel zum Trocknen von Gasen und Flüssigkeiten durch Entzug von Wasser (z.B. Entwässerung von Ölen, Petroleum und Paraffin)
- Akkumulatorsäure für Autobatterien
- Ätzen von Halbleitern (z.B. Elektronikindustrie)
- Zusatzmittel für Wasser bei der Elektrolyse (aufgrund ihrer Leitfähigkeit)
- Lösungsmittel beim Abrauchen (Entfernen flüchtiger Verbindungen aus festen Substanzen durch Erhitzen).
- Bestimmung der Viskositätszahlen von Polyamid
- Hilfsstoff in der Lebensmittelindustrie zur Herstellung von modifizierter Stärke und Casein
- Trinkwasseraufbereitung
- Aufschließung von Erzen (z.B. bei Titan(IV)-oxid oder Uran)
Warum ist Schwefelsäure massiv von Verknappung betroffen?
Schwefelsäure kann momentan nicht mehr in den notwendigen Mengen zur Verfügung gestellt werden. Die Ursachen dieser extremen Verknappung sind in einem Zusammenspiel verschiedener Komponenten und erschwerender Rahmenbedingungen zu finden.
1. Ölfördermenge durch Covid-19 massiv reduziert
Eine wesentliche Komponente, die sich äußerst nachteilig auf die Verfügbarkeit von Schwefel auswirkt, ist der Einbruch der Ölfördermengen. Mit dem starken Rückgang des Flug- und Straßenverkehrs im Zuge der weltweiten Shutdowns ist der Treibstoff- und Kerosinverbrauch drastisch gesunken. Raffinerien setzen entsprechend weniger um, wodurch nicht nur die Schwefelproduktion empfindlich gestört ist, sondern
sämtliche Basischemikalien einer starken Verknappung unterliegen. Die Havarie im Suezkanal hat die Verfügbarkeit von Rohöl in Europa ebenfalls belastet und führt indirekt zu einer Preissteigerung von Schwefel.
2. Raffinerien in Europa gleichzeitig auf Maintenance gestellt
Die ohnehin schon angespannte Situation wird zusätzlich durch Wartungsarbeiten in Raffinerien verschärft, die ungünstigerweise bei allen Refinern gleichzeitig durchgeführt werden. Die Anlagen sind derzeit für drei bis sechs Wochen stillgelegt, wodurch die Verfügbarkeit von Schwefel weiter unter Druck gerät.
3. Raffinerien wechseln zu süßem Rohöl
Die Qualität des Rohöls ist zum einen bestimmt durch die Dichte, zum anderen spielt vor allem der Schwefelgehalt eine Rolle. Es gilt: Je geringer Dichte und Schwefelgehalt, desto besser für Raffinerien. Leichte und süße schwefelarme Rohölsorten sind in der Herstellung kostengünstiger und einfacher zu raffinieren als schweres, saures Rohöl. Das ist auch der Grund, warum schwefelreiche Rohölsorten lange günstiger in der Beschaffung waren.
Dieser Preisvorteil ist weggefallen, süßes und saures Rohöl kostet gleich viel. Raffinerien haben daher begonnen, bevorzugt süßes Rohöl einzukaufen, welches aber nur ein Fünftel des Schwefelgehaltes im Vergleich zu saurem Rohöl beinhaltet. Damit hat sich die Schwefelmenge, die als Beiprodukt beim Fraktionieren von Erdöl gewonnen wird, auf rund ein Fünftel reduziert.
Süßes Rohöl hat aber nicht nur verarbeitungstechnische, sondern auch umwelttechnische Vorteile. Denn je weniger Schwefel im Öl ist, desto freundlicher wird es für die Umwelt. Da sich Raffinerien zunehmend als grüne Unternehmen positionieren wollen, wird auch aus diesem Grund weniger saures Rohöl verarbeitet.
4. Erdöl- und Erdgasförderung in Europa geht generell zurück
Ein weiterer Aspekt, der jedoch erst in einigen Jahren auf die Schwefel Verfügbarkeit Einfluss nehmen wird, ist der Rückgang der Erdöl- und Erdgasförderung in Europa. In absehbarer Zeit werden speziell in Mitteleuropa die Ressourcen erschöpft sein. Zukünftig wird man Flüssigschwefel zu höheren Preisen aus weiter entfernten Raffinerien beziehen müssen. Eine andere Option wäre festen Schwefel einzukaufen und zu flüssigen Schwefel umzuwandeln, um daraus Schwefelsäure produzieren zu können. Auch das ist ein Zukunftsmodell, welches aber voraussichtlich erst in ein einigen Jahren relevant werden wird.
Fazit: Verknappung von Schwefelsäure wird noch andauern
Da die Wartungsarbeiten der Raffinerien in einigen Wochen abgeschlossen sind, ist für Mai mit einer leichten Entspannung der Lage zu rechnen. Was aber aus heutiger Sicht noch einige Zeit anhalten wird, ist die Covid19 Situation, d.h. es wird voraussichtlich immer noch zu wenig Treibstoff und Kerosin produziert, um den Schwefel Engpass zu beseitigen.
Ebenso werden Raffinerien weiterhin das süßere Rohöl forcieren, solange saures und süßes Rohöl gleich teuer bleiben. In Summe sprechen die genannten Faktoren somit nicht dafür, dass es rasch wieder günstigen Schwefel in ausreichenden Mengen für die Schwefelsäureproduktion geben wird.
 www.donauchem.at
www.donauchem.at
Weiterführende Links/Downloads:
Donau Chemie AG - Schwefelsäure-Produzent
Whitepaper Schwefelsäure