Sulphuric acid is currently affected by a shortage not seen since the Second World War. Currently, there are only limited quantities of liquid sulphur, which is a by-product of the desulphurisation of crude oil in refineries and is mainly used in Europe for the production of sulphuric acid.
We take this as an opportunity to take a closer look at this basic chemical and its enormous importance for the economy. Furthermore, we look at the reasons for the poor availability and give an assessment for the coming months.
What is sulphuric acid?
Sulphuric acid is one of the most technically important chemicals in the world and one of the most widely produced basic chemicals. The reason for this lies in the properties of the versatile acid.
- Sulphuric acid is a colourless, odourless and oily liquid.
- It is one of the most powerful mineral acids.
- Sulphuric acid can be mixed with water in any ratio and gives off high amounts of heat.
- In aqueous solutions, sulphuric acid is a strong, dibasic acid.
- Hot sulphuric acid can dissolve precious metals.
- Sulphuric acid attracts moisture from the air (hygroscopic).
- In high concentrations, sulphuric acid has a dehydrating and strongly oxidising effect.
- Concentrated sulphuric acid is highly corrosive and destroys organic substances such as sugar, cotton or skin, forming carbon.
Characterisation of sulphuric acid 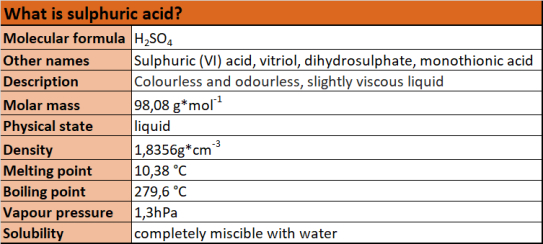
Which manufacturing processes existed in the course of history?
Sulphuric acid has been known as vitriol for a long time. The vitriol process is the oldest process for producing sulphuric acid. It is based on the thermal decomposition of naturally occurring sulphates, the so-called vitriols.
It was first mentioned in the writings of the 8th century alchemist Dschābir ibn Hayyān and described in more detail in the 13th century by the alchemists Albertus Magnus and Basilius Valentinus. From the 16th century onwards, due to the increasing demand for sulphuric acid, the vitriol process was used on an industrial scale.
Johann Rudolph Glauber was the founder of the world's first sulphuric acid manufactory, which produced sulphuric acid using this process in Nordhausen (Harz) around 1650.
Because of the high temperatures required, however, the process was quite expensive and was quickly superseded by the lead chamber process after it was developed. In this production method, the sulphuric acid was produced by burning sulphur and saltpetre in lead containers.
After Nicolas Clément-Désormes and Charles Bernard Désormes discovered in 1793 that the amount of saltpetre could be significantly reduced by using air, the lead chamber process could also be used on a large scale. Modern processes are the contact process and the double contact process that was further developed from it.
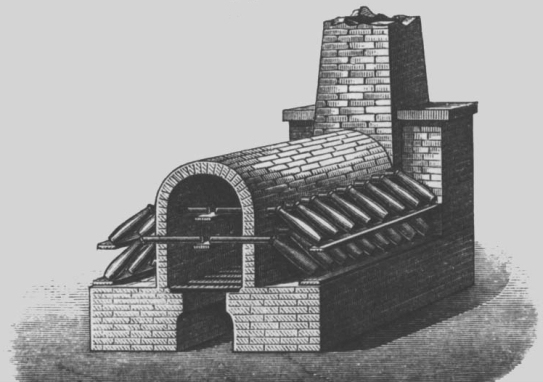
Picture: Galley furnace as used in the past for the vitriol process.
How is sulphuric acid produced today?
Crude oil and natural gas are often contaminated with sulphur. Before they are processed, they are desulphurised in the refinery. The liquid sulphur produced here can be used to make sulphuric acid. In large-scale applications, sulphuric acid is now produced in a continuous process using the so-called double-contact method.
In Austria, Donau Chemie AG is the only producer that manufactures sulphuric acid for the free market. In our plant in Pischelsdorf, the production process of high-purity sulphuric acid passes through a total of six steps:
Sulphur furnace: combustion of the liquid sulphur with atmospheric oxygen to form gaseous sulphur dioxide (SO2). The waste heat generated in the process is used to produce steam.
- Contact tower 1st contact: Oxidation of SO2 (+O2/oxygen gas) by means of a catalyst to gaseous sulphur trioxide (SO3). Residual SO2 remains, conversion of approx. 85 percent of the sulphur used. (Contact: catalyst bed for conversion of SO2 to SO3).
- Intermediate absorber: conversion of SO3 with concentrated liquid sulphuric acid (H2SO4), with simultaneous addition of water.
- Contact tower 2nd contact: Oxidation of the remaining SO2 (+O2) by means of a catalyst to SO3. Conversion of approx. 99.8 percent of the sulphur used. (Contact: catalyst bed for conversion of SO2 to SO3).
- Final absorber: Conversion of the remaining SO3 with concentrated H2SO4 with simultaneous addition of water. The resulting waste heat from the two absorbers is used for hot water production.
- Drying tower: Drying of the air with concentrated H2SO4 for sulphur combustion and adjustment of the final H2SO4 concentration to 96 per cent with demineralised water.
Illustration of the double contact process
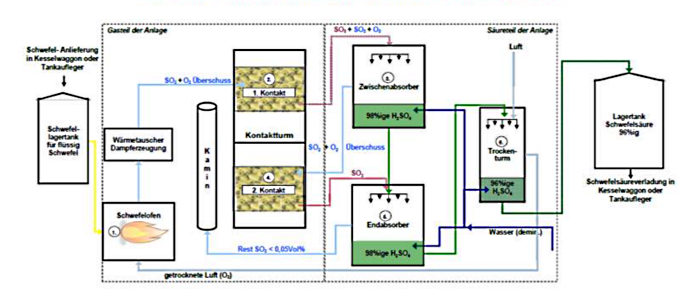
© Donau Chemie AG
Why is sulphuric acid so important and critical for the chemical industry?
The importance of sulphuric acid is often underestimated, even though it is the most produced and used chemical worldwide. In 2020, the global sulphuric acid market reached a volume of almost 284.4 million tonnes.
As a key product of the chemical industry, sulphuric acid is used in very large quantities and directly or indirectly in almost all chemical processes. In particular, it is an irreplaceable product for basic processes.
Subsequently, this means that if it were not available, it would be almost impossible or simply unprofitable to carry out a large number of basic and extremely important industrial processes. Many products cannot be produced without the "blood of chemistry", or only at much greater expense.
What role does sulphuric acid play in the economy?
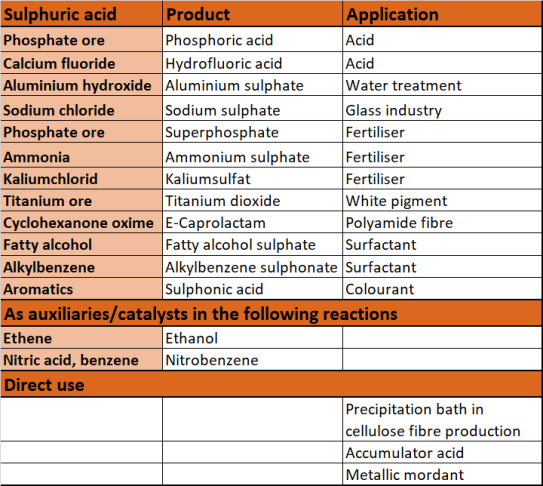
The largest quantity of sulphuric acid is used in the production of fertilisers, especially for the production of superphosphates, ammonium phosphate and ammonium sulphate. It is also used in the production of dyes, pharmaceuticals, detergents, plastics or explosives. In addition, sulphuric acid is of great importance for many organic-technical syntheses as a reaction partner, reaction aid or catalyst. It is also used as a drying agent or in the processing of metals.
The most important applications of sulphuric acid are:
- Production of sulphates (e.g. for detergents, glass and paper industry).
- Production of other acids (e.g. hydrochloric acid, nitric acid, hydrofluoric acid or phosphoric acid)
- Production of fertilisers
- Production of surfactants (e.g. for detergents and cleaning agents, cosmetics, pharmaceutical products)
- Production of titanium dioxide (e.g. for white pigments in many paints, plastics and lacquers)
- Catalyst (e.g. for esterification)
- Reaction aid for organic syntheses (nitrating acid)
- Metal treatment (e.g. pickling of metals)
- Desiccant for drying gases and liquids by removing water (e.g. dehydration of oils, petroleum and paraffin)
- Accumulator acid for car batteries
- Etching of semiconductors (e.g. electronics industry)
- Additives for water in electrolysis (due to their conductivity)
- Solvents in fuming (removing volatile compounds from solid substances by heating).
- Determination of the viscosity numbers of polyamide
- Auxiliary in the food industry for the production of modified starch and casein
- Drinking water treatment
- Digestion of ores (e.g. for titanium(IV) oxide or uranium)
Why is sulphuric acid massively affected by shortages?
Sulphuric acid can currently no longer be made available in the necessary quantities. The causes of this extreme shortage are to be found in an interplay of various components and aggravating framework conditions.
1. Oil production massively reduced by Covid-19
One major component that has had an extremely negative impact on the availability of sulphur is the collapse in oil production volumes. With the sharp decline in air and road traffic in the wake of the global shutdowns, fuel and paraffin consumption has fallen dramatically. Refineries are turning over correspondingly less, which has not only severely disrupted sulphur production, but all basic chemicals are subject to severe shortages. The accident in the Suez Canal has also affected the availability of crude oil in Europe and indirectly leads to an increase in the price of sulphur.
2. Refineries in Europe put on maintenance at the same time
The already tense situation is further aggravated by maintenance work in refineries, which is unfavourably being carried out at all refiners at the same time. Plants are currently shut down for three to six weeks, putting further pressure on sulphur availability.
3. Refiners switch to sweet crude oil
The quality of the crude oil is determined on the one hand by the density, and on the other hand the sulphur content plays a major role. The rule is: the lower the density and sulphur content, the better for refineries. Light and sweet low-sulphur crude oil grades are cheaper to produce and easier to refine than heavy, sour crude oil. This is also the reason why high-sulphur crude oil grades were cheaper to procure for a long time.
This price advantage has disappeared; sweet and sour crude oil cost the same. Refineries have therefore started to prefer buying sweet crude oil, which, however, contains only one fifth of the sulphur content compared to sour crude oil. This has reduced the amount of sulphur obtained as a by-product in the fractionation of crude oil to about one-fifth.
Sweet crude oil, however, not only has processing advantages, but also environmental ones. After all, the less sulphur there is in the oil, the friendlier it is for the environment. As refineries increasingly seek to position themselves as green companies, less sour crude oil is being processed for this reason as well.
4. Oil and gas production in Europe is generally declining
Another aspect that will only have an impact on sulphur availability in a few years' time is the decline in oil and gas production in Europe. In the foreseeable future, resources will be exhausted, especially in Central Europe. In the future, liquid sulphur will have to be purchased at higher prices from more distant refineries. Another option would be to buy solid sulphur and convert it into liquid sulphur in order to produce sulphuric acid. This is also a model for the future, but it will probably only become relevant in a few years' time.
Conclusion: Shortage of sulphuric acid will persist
Since the refineries' maintenance work will be completed in a few weeks, a slight easing of the situation can be expected for May. However, from today's perspective, what will continue for some time is the Covid19 situation, i.e. there is still likely to be too little fuel and paraffin produced to eliminate the sulphur shortage.
Similarly, refiners will continue to push sweeter crude as long as sour and sweet crude remain equally expensive. In sum, the above factors do not suggest that cheap sulphur will quickly become available again in sufficient quantities for sulphuric acid production.
 www.donauchem.at
Related links/downloads:
Donau Chemie AG - Acetic acid producer
Whitepaper Acetic Acid (german)
www.donauchem.at
Related links/downloads:
Donau Chemie AG - Acetic acid producer
Whitepaper Acetic Acid (german)