Foam is quite welcome in beer and useful for shaving and fire-fighting. In many industrial applications, however, large amounts of foam are an annoying by-product and must be removed. Accordingly, defoamers are widely used in industry. We give an overview of foam, its stability, characterisation and impact on different industries as well as the mode of action and types of defoamers.
What is foam and how does it build up?
Liquid foam consists of small gas bubbles (usually air) separated by liquid walls (lamellae). Immediately after their formation, the gas bubbles rise to the surface in the liquid phase. In order for stable foam to form, surface-active substances (surfactants) must be present in the liquid phase. Pure liquids (without surface-active substances) do not foam because the foam bubbles burst when they reach the liquid surface.
Foam-stabilising effects of surfactants
In general, surfactants are characterised by the fact that they contain hydrophilic (water-loving) and hydrophobic (water-repelling) or lipophilic (fat-loving) chemical groupings in the molecule. Due to this structure, the molecules are surface-active, i.e. they arrange themselves in the liquid/gas interface in such a way that the hydrophobic part does not come into contact with water. The associated reduction of the interfacial tension creates the conditions for a stable foam.
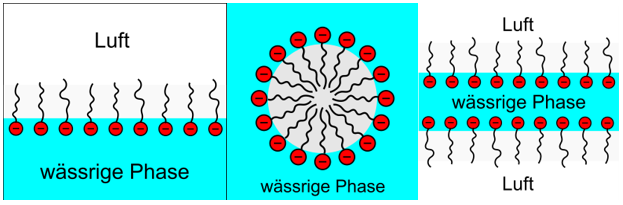
The arrangement of the surfactants can essentially take place in three ways:
Source:
Wikipedia
- The surfactants accumulate at the interface between water and air and thus reduce the surface tension of the water (Fig. left).
- The surfactants can also "dissolve" in water by clumping together and forming so-called micelles (Fig. middle).
- When the liquid forms a thin film, the surfactants accumulate in two surface layers, with the hydrophilic ends protruding into the solution (fig. right).
Liquid foam is thus formed whenever the surface tension of the water is reduced by the accumulation of surfactants and air is introduced by whipping, blowing or similar methods. The resulting bubbles are then stabilised by the formation of a surface layer.
Spherical foam - Polyhedral foam - Two-dimensional foam
From a thermodynamic point of view, foams are unstable multiphase systems because their formation is constantly changing. If we look at the life cycle of a foam, we find that it loses liquid over time and thus changes its structure.
Accordingly, two types of foam are distinguished: spherical foam and polyhedral foam.
1. Spherical foam
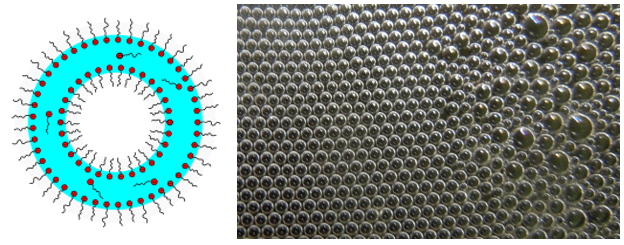
Shortly after its formation, the foam still contains a relatively large amount of liquid. The individual gas bubbles are spherical with a thick layer of lamellae and do not yet deform each other. Such a foam is therefore also called "wet foam" or "spherical foam".
The presence of surfactants is not essential for the formation of spherical foam. In this case, the stability and lifetime of the foam depend on the viscosity and density of the liquid. In low viscosity liquids such as water, the foam disintegrates within seconds. If you shake a bottle of water, for example, rising bubbles form which burst when they penetrate the surface of the liquid.
©
Kempf EK,
Order and Chaos,
CC BY 4.0 (Abb.rechts)
2. Polyhedral foam
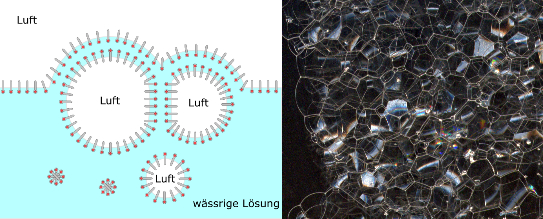
When the spherical foam stabilises on the surface of the liquid, the liquid flows out of the lamellae downwards due to gravity (drainage effect). The lamellae become thinner, the gas bubbles move closer together, deform each other and become polyhedra. This foam is called "dry" or "polyhedral" foam.
As soon as the lamellae in the upper area become too thin, the foam bubbles break. However, various counterforces can slow down the drainage process and prevent the lamellae from breaking. Causes for this are the electrostatic repulsion of the surfactant molecules, the Marangoni effect, but also external influences such as temperature and viscosity.
©
Roland.chem,
Schaumbildung,
CC BY-SA 3.0/
André Karwath, Foam - big,
CC BY-SA 2.5
If the polyhedra of polyhedral foams are next to each other, then these polyhedral foams are called "two-dimensional foam". This type of foam is created by compressing the foam between two plates, whereby the distance between the plates must be smaller than the diameter of the smallest bubble. Two-dimensional foam occurs in many production processes and can build up very quickly.
 © Klaus-Dieter Keller
© Klaus-Dieter Keller
In which industries arise problems with large amounts of foam?
Foam formation in liquids often causes disruptions in process plants because the foam penetrates into plant areas where the liquid is not supposed to go. While surface foam is easily recognised by the foam bubbles, enclosed air or gas bubbles are usually only recognisable by their disruptive effects on the operating process.
Paper industry
In the paper industry, extreme foaming can occur throughout the production process - starting with pulp and ending with paper production. Foam causes cavitation of the pumps as well as measurement errors in levels and volumes, and affects the quality of the end product through holes and defects in the paper. In applications such as paper coating, air bubbles disrupt the finishing process and impair the coating quality. Depending on the plant and application, suitable defoamers are therefore needed for paper processing (size press, coating), for coating colours and, above all, for waste water treatment.
Chemical synthesis
In chemical synthesis (esterifications, conversions, amidations, etc.), the addition of defoamers is necessary in the process for strongly foaming raw materials. Or also in polymerisations, as the wetting agents (surfactants) contained have the property of foaming strongly.
Fermentation
Foam formation in fermentative processes for the production of e.g. bioethanol, citric acid or lactic acid is caused by foam-active substances in the fermenter broth, escaping gases/air and turbulence in the fermenter. Amino acids and proteins formed by microorganisms during fermentation can also lead to considerable foaming. The addition of defoamers prevents fermenters from foaming over, which would lead to considerable contamination in addition to the loss of product quantities.
Waste water treatment & drinking water treatment
Industrial wastewater, wastewater from businesses and agriculture or wastewater from private households usually contain surface-active substances such as surfactants that cause foaming. In the course of biological purification in the sewage treatment plant, the work of the bacteria can also produce gases and consequently foam. Depending on the application, appropriate defoamers are required for the aerobic or anaerobic area of wastewater treatment as well as those for special industrial process wastewater.
Paint and varnish industry
Foam can already occur during the production and filling of paints and varnishes and can lead to containers not being filled optimally. Most problems, however, occur during application. Foam causes surface disturbances and reduces the protective function of the coating. For these reasons, a defoamer is an integral part of the formulation in most paint and varnish systems.
Construction industry
When drying cement, concrete, plasters or self-levelling products, bursting foam bubbles cause damage such as holes and other surface impairments. To prevent this, powder defoamers are used. Self-levelling products are their main application. They are mainly used in screeds and contain cement, lime, thickeners, binders, superplasticisers, retarders, accelerators and defoamers. The defoamer destroys the foam in advance and has a deaerating effect. At the same time, the deaerator improves the flowability of the cement after mixing.
Food industry
In the food processing industry, a wide variety of causes can lead to considerable foam formation. Disruptions to operating processes and losses of considerable production quantities are the result. Defoamers support process stability and prevent contamination and other negative effects. One of the biggest challenges for defoamers is sugar production, for example, as the use of defoamers is required in all process steps - from beet washing to molasses transport.
How defoamers work
The term "defoaming" is used to describe the removal of gas bubbles. In certain cases, however, a distinction should be made between "defoaming" and "deaeration":
- Defoamers are only effective on the surface, where they destabilise the foam bubbles and cause them to burst. Foam inhibitors and foam destroyers fall into this category. These form a closed film at the interface of the liquid, which accelerates the outgassing or enables it by destroying the gas bubbles.
- Deaerators, on the other hand, bring gas particles together to form bubbles which subsequently rise to the surface and can thus escape. Foam deaerators must therefore have an effect on the entire medium and not just on the surface.
Basically, the mode of action of defoamers can thus be divided into three classes:
- Foam destruction: A defoamer of this class destroys the foam on the surface. The foam destroyer is either sprayed on from above or allowed to trickle down from above.
- Foam prevention: Antifoam additives have the property of immediately breaking down foam formations and preventing new foam from forming. The foam inhibitor is usually introduced into the medium to be defoamed via a dosing point, whereby it is important to consider well in advance at which point it is best to dose. The selection of the right dosing point is extremely important for the optimal effectiveness of the defoamer.
- Foam deaeration: Defoamers with a strong deaerating effect are especially important for paint and lacquer systems. Deaerators accelerate the rate at which air bubbles (microfoam) rise in wet paints and varnishes so that the air bubbles can leave the medium before it dries. Without deaerators, for example, tiny holes would appear in the surface after an anticorrosive paint has dried, again providing a surface for corrosion to attack.
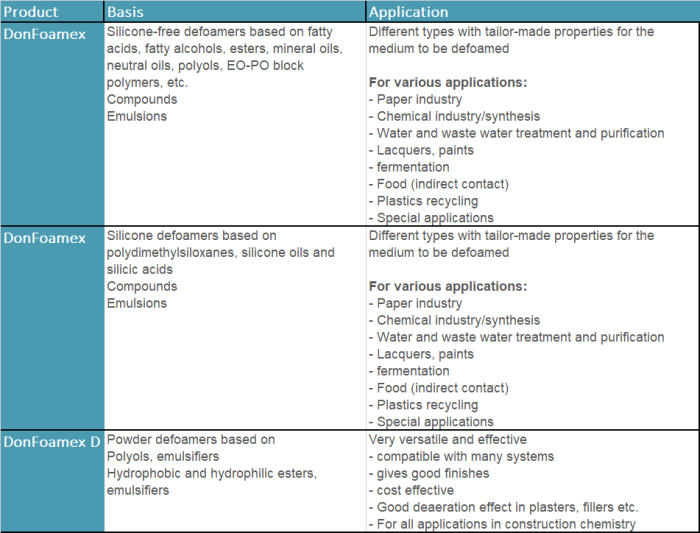
Types and chemical classification of defoamers
Depending on their composition, defoamers have a more or less strong deaerating effect, prevent foam formation or destabilise existing foams. In general, defoamers/deaerators can be divided into four main groups:
- Silicone-free defoamers: defoaming fluids based on mineral oils, polyols, EO-PO block polymers, fatty acids/fatty alcohols, esters, ethers, etc.
- Silicone defoamers: Defoaming liquids with particularly low surface tension, which contain dimetylsiloxanes in different variants as the main active substance. In addition, various silicic acids or silicone oils can be added.
- Other organic defoamers: Defoaming liquids based on simply defined organic substances that have a defoaming effect in various applications, e.g. triisobutyphosphate/tir-n-butyl phosphate and other phosphate esters.
- Powder defoamers: Powdery/solid defoamers for solid building products that are mixed with liquid, e.g. wallpaper paste, tile adhesives or fillers.
Below is a brief overview of which defoamers Donauchem offers (product name:
DonFoamex) and in which applications they are used:
Conclusion: Finding the right defoamer
It is not possible to say in general which defoamer is suitable for which application - this should be determined in each case by preliminary tests. In wastewater treatment, for example, emulsions of different silicone content or non-silicone soluble compounds of different concentrations are used. In the detergent and cleaning agent sector, on the other hand, defoamers with different silicone contents and viscosities are required, which can occur as emulsions, soluble compounds, powders, liquids, etc.
To find out which product is best suited for your application please contact
Donauchem's sales team.
 www.donauchem.at
www.donauchem.at