Solvents are substances or compounds that are usually present in liquid form and have the ability to dissolve other substances, such as gases, other liquids or solids, without undergoing a chemical reaction with the substance to be dissolved.
Solvents/solvents of Donauchem GmbH
The product group of solvents is one of the most important product groups at Donauchem GmbH and is of great importance within a long series of industrial applications, but also applications in the pharmaceutical and biotechnology industry.
Thanks to our well-developed, large and flexible warehouse at our plant in Pischelsdorf, we are able to react very quickly to changing situations. In a total of 11 storage or underground storage tanks with a filling volume of 40-1000 m3 for standard solvents, we can supply the most important industries in Austria with solvents.
We offer solvents both in tanker trucks and as bulk deliveries, i.e. in packaged form. Our state-of-the-art filling facilities enable us to fill solvents in container sizes of 25-1000L; smaller container sizes would also be possible upon special customer request.
Our portfolio includes the most important standard solvents (alcohols, ketones, benzines and special benzines, aromatics, esters etc.) and also all special solvents. You can find more about Donauchems solvents portfolio here.
Solvents in everyday life
The term solvent is mainly known from the field of varnishes, paints, adhesives etc. and is characterised for most people by unpleasant odours or vapours. In many cases, products such as various household or industrial cleaners also contain a not inconsiderable proportion of solvents.
Solvent-free formulations are those in which the solvent content is max. 0.5%.
Solvents based on renewable raw materials are becoming increasingly important and are usually sold on the market under the name "green solvents".
Chemistry of solvents
The solvent does not actively participate in the reaction, but it is nevertheless an extremely important component of the respective reaction and decisively responsible for the dissolving process.
The effects of the solvent on the reaction can vary and depend on the reaction partners and the type of reaction carried out. In addition, the concentrations of the substances and the temperature during the chemical reaction also play an important role. In any case, these properties must always be taken into account when selecting a suitable solvent.
Most important tasks of the solvent during chemical reactions:
- convective heat and mass transport
- stabilisation of transition states of the reaction
- dilution to avoid side reactions
Some important procedures in cleaning and process operations where solvents are used:
- Precipitation
- Crystallisation
- Recrystallisation
- Extraction
- Chromatography
Classification/classes of solvents
By default, solvents are primarily divided into various classes according to their physical properties:
- Boiling point
- Permittivity (polarisation ability)
- laFmmpunkt
- Volatility
- Viscosity
- Polarity
Aprotic solvents
Aprotic solvents are solvents that do not have a functional group from which protons can be split off.
Aprotic non-polar: these solvents are readily soluble in each other, such as alkanes, alkenes, benzene and other aromatics, carboxylic esters, etc. They are very lipophilic and therefore not soluble in water and other polar solvents such as alcohols.
Aprotic polar: Solvents which contain strongly polar functional groups in the molecule such as the carbonyl group, the nitro group or the nitrile group.
Protic solvents
If the molecule of the solvent has a functional group from which hydrogen atoms in the molecule can be split off as protons (dissociation), it is called a protic solvent. The most important protic solvent is water. Other representatives of this group are: Alcohols, carboxylic acids and mineral acids.
Table of important solvents and physical data
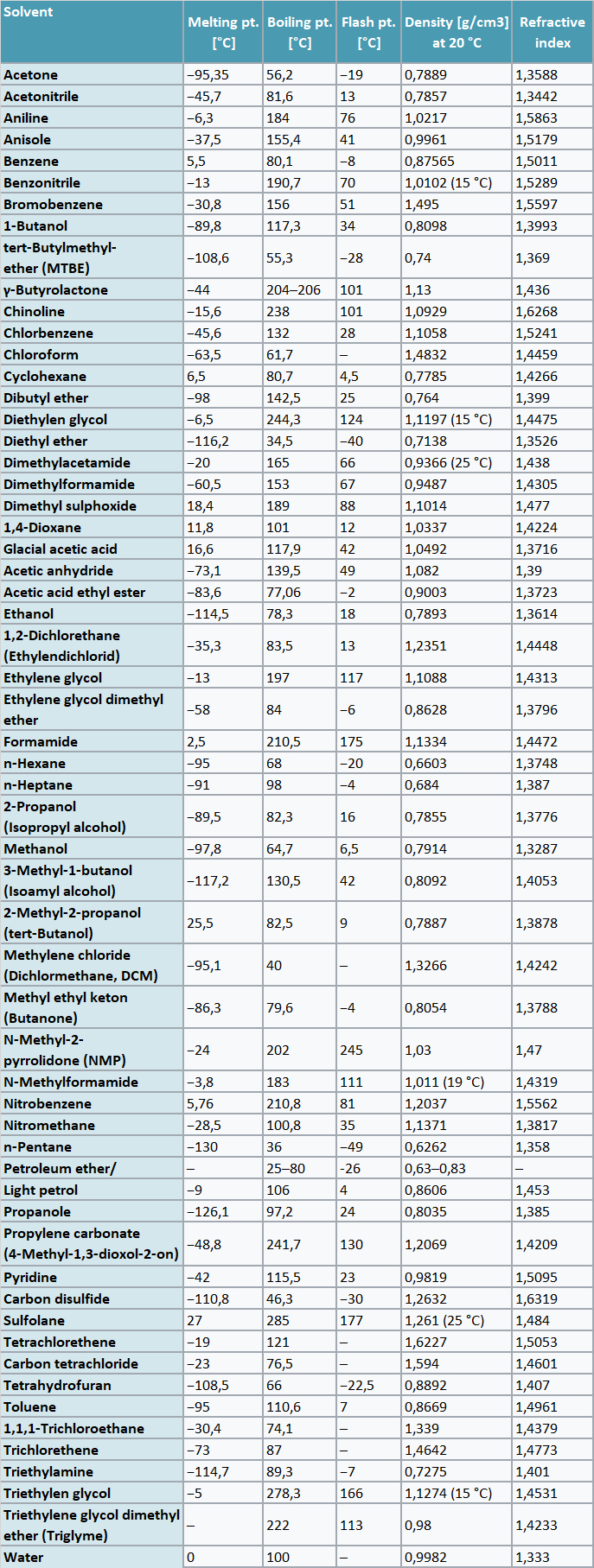
Source:
Wikipedia
The
product portfolio of Donauchem GmbH includes a range of solvents.
For further information or quotations
please contact us.
 www.donauchem.at
Related Links:
Chemie.de 28.März 2022
www.donauchem.at
Related Links:
Chemie.de 28.März 2022