Water is not only our most important food, but many life forms on our planet are largely made up of water. The human body, for example, contains between 50-65% water, and many higher animals have a similarly high proportion of water in their bodies.
The most important of all liquids fulfils several vital functions at once, which form the basis for the cycle of life. For it is through the properties of water that many processes in nature can take place in the first place.
Water as a chemical compound
Water has the chemical formula H
2O, consisting of the elements oxygen (O) and hydrogen (H).
It is referred to as water in its liquid state, as water vapour in its gaseous state and ice refers to the solid state of water. Naturally occurring water is not pure water, because proportions of minerals, salts, gases and organic compounds are dissolved in it. All biological processes on our planet only take place thanks to liquid water.
Structural formula:
The basic building block of life
According to current knowledge, life originated in water. Autotrophic sulphur bacteria (prokaryotes) produce organic carbon compounds and water from hydrogen sulphide and carbon dioxide under the supply of light energy:
18 H
2S + 6 CO
2 
C
6H
12 + 12 H
2O + 18 S
As successors, blue bacteria (cyanobacteria) and autotrophic eukaryotes produced glucose and oxygen from water and carbon dioxide with the addition of light:
6 CO
2 + 12 H
2O

C
6H
12O
6 + 6 O
2 + 6 H
2O
d in the atmosphere. This made the generation of energy through cellular respiration (dissimilation) possible:
C
6H
12O
6 + 6 O
2 
6 H
2O + 6 CO
2
A prerequisite for the ability to deal with toxic oxygen (oxidation of sensitive biomolecules) were enzymes such as catalase, which has a structural similarity to the oxygen-transporting haemoglobin. Aerobic purple bacteria were perhaps the first to use toxic oxygen for the energy-producing degradation of organic matter. According to the endosymbiont theory, at that time still anaerobic eukaryotes absorbed the aerobic prokaryotes (probably purple bacteria).
Water thus became the medium of fundamental biochemical processes (metabolism) for energy production and storage:
- Photosynthesis, dissimilation
- Glycolysis
- Citric acid cycle
- Fat breakdown
- Protein breakdown
- Urea cycle
Properties of water
Water occurs in two isomers (para- and ortho-water), which differ in the nuclear spin of the two hydrogen atoms.
Water forms a so-called dipole because oxygen has a higher electron negativity than hydrogen. Due to the dipole formation, hydrogen molecules have pronounced intermolecular forces of attraction and cluster together via hydrogen bridge formations.
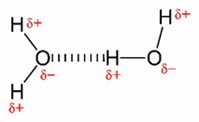
Fig.: Representation of two water molecules with partial charges marked in red, connected by a dashed hydrogen bond. Source:
Wikipedia
Interesting facts about water
- Water has a density of around 1000 kg/m³ (originally the definition of the kilogram), more precisely: 999.975 kg/m³ at 3.98°C. The term density anomaly is used to describe the property, based on hydrogen bonding, that water has the highest density at this temperature and continuously increases in volume when cooling below this temperature and even abruptly when freezing, i.e. it loses density so that ice floats on water,
- It has a viscosity of 1.0019 mPa-s (0.010019 poise) at 20 °C,
- It has one of the highest specific heat capacities of liquids at room temperature (75.366 J-mol-1-K-1 corresponding to 4.18 kJ-kg-1-K-1 at 20 °C,
- It has one of the highest surface tensions of any liquid (only mercury has a higher one); for water, it is 72 mN/m at +20 °C in humid air, facilitating droplet formation,
- It has one of the largest specific enthalpies of evaporation of all liquids (44.2 kJ/mol corresponding to 2453 kJ/kg at 20 °C; hence the cooling effect during transpiration) as well as a high enthalpy of fusion (6.01 kJ/mol corresponding to 333 kJ/kg; so that salt water shows only a slight decrease in freezing point compared to pure water),
- It has a low thermal conductivity (0.6 W/(m K) at 20 °C).
- Depending on the isotopic composition of the water molecule, a distinction is made between normal "light water" (two atoms of hydrogen: H2O), "semi-heavy water" (one atom of hydrogen and one atom of deuterium: HDO), "heavy water" (two atoms of deuterium: D2O) and "superheavy water" (two atoms of tritium: T2O), with HTO and DTO being other molecules with mixed isotopes.
- Water can form a water bridge between two glass vessels under high voltage.
Water chemistry
Water is a solvent for many hydrophilic gaseous substances, hydrophilic organic substances, minerals, various ionic compounds and other substances.
In general, the following types of water are distinguished in water analysis:
- Drinking water
- Mineral water
- Medicinal water
- Table water
- Freshwater/seawater/saltwater/breakwater
- Ultrapure water
- Demineralised water
- Distilled water
- De-ionised water
- Process water
- Waste water, (household waste water, agricultural waste water, industrial waste water)
- Rainwater
- Groundwater
- Surface water (flowing and standing water)
Water - our most important resource
If there were no water in our planet's atmosphere, the average temperature on Earth would be much lower, because the sun's heat would be reflected directly into space.
The exchange of water in inshore waters is caused by temperature fluctuations due to the respective season. And the distribution of heat on Earth is also based on the factor that large ocean currents bring warm water to colder areas. All water on earth is in a constant cycle and can therefore neither be consumed nor recreated.
To emphasise the fundamental importance of water, it was defined as a fundamental human right in a resolution of the EU Parliament in 2005. Unfortunately, however, it is not available to all people in sufficient quantity and quality.
Industrialised countries in particular are implementing improved use of their water resources, thereby minimising water consumption. The flip side of this development, however, is that production facilities are being relocated to other countries, which shifts both the water consumption for these productions and the associated environmental pollution to these countries.
Water cycle
The water cycle begins when water evaporates from the oceans. The water vapour then falls back onto the earth's surface in the form of rain, hail or snow, depending on the region and season. Ultimately, it flows back into the sea in the rivers - the cycle starts all over again.
Every second, about one billion litres of water evaporate on our planet. Nevertheless, only 0.77 per cent of the total water takes part in the water cycle. The majority is bound up in oceans or ice caps.
What happens to rainwater?
- In the surface water cycle, rainwater flows mostly as surface water in rivers into the sea, where it evaporates again at the surface.
- The underground water cycle is fed by the water that enters deeper into the ground. This also includes groundwater.
- Water from precipitation is also absorbed by plants and released back into the atmosphere through transpiration.
- Some water also evaporates at the surface before it can enter another water cycle.
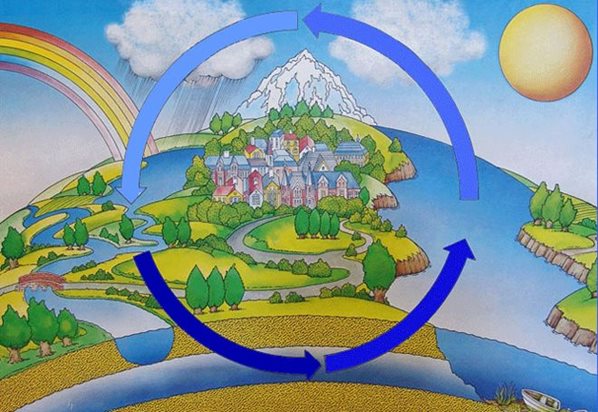
Fig.: The water cycle - Source:
Wasserwerk.at
Water use
Water accompanies us in almost all areas of life. Whether as a necessary food or for the growth of plants and thus food, it also plays a role in industry that is not be underestimated.
It is used as a cleaning agent, coolant, production medium, energy supplier or means of transport in various areas of application. But here in particular it is important to ensure that the water is returned to the natural cycle in a clean state. Pollution must be removed by means of various filtration methods before discharge in order to avoid environmental consequences such as fish mortality. And the temperature of this water must not disturb the natural cycle either.
Groundwater abstraction by sector
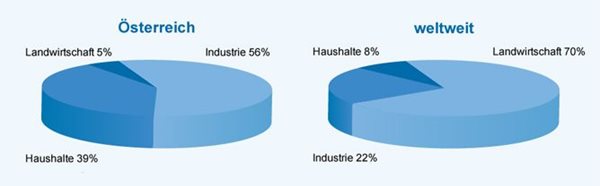
Source:
Wasserwerk.at
Water as a solvent
Water ist one of the most important solvents. Because of its polarity and ability to form hydrogen bonds, water can dissolve many types of molecules. This ability is the basis for various chemical reactions.
We also use water as a solvent in everyday life: for example, when preparing tea or coffee, which give aroma and colour to hot water. Or adding sugar to that cup of tea, which dissolves completely in the water.
Most of these reactions, which are important for life, take place in a watery environment inside the cells. In the body, water acts as a transport medium, as it dissolves nutrients and other vital substances and transports them through our bodies. For aquatic organisms, for example, it is vital that oxygen dissolves in water, which they absorb through their gills or directly through their skin.
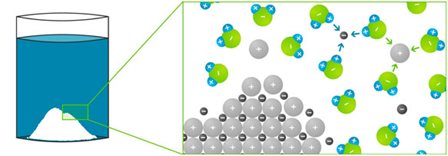
Source:
Klassewasser.de
Synthesis, electrolysis and chemical use
Water was first synthesised as a chemical compound in the 18th century. A mixture of hydrogen and air was made to explode (oxyhydrogen reaction).
The electrolysis of water yields hydrogen, which is considered to be an important future energy source. In the process, water is broken down into its components in the Hofmann water decomposition apparatus. Reaction diagram:
2 H
2O

2 H
2 + O
2
Why do bubbles form in boiling water?
The effect of heat causes the water molecules to move faster. When 100 °C is reached, the aggregate state changes from liquid to vapour. Steam has a volume that is about 1600 times greater and a lower density: the water begins to boil, whereby the steam bubbles are cooled by layers of water that are not yet so hot and condense back into liquid water. When the entire volume of water finally reaches the temperature of 100 °C, the now large steam bubbles reach the surface: the water boils.
Pressure and temperature are the determining factors for the solubility of gases in water. Gas bubbles, which become visible even at slight heating, consist of dissolved gases. This is due to the lower solubility of gases in water when heated. Water that has been in a pressurised pipe or bottle for a while has often dissolved an excess of gases. Therefore, even the removal of external pressure is enough for gas bubbles to form - preferably on germs on the wall - and to adhere up to a size of 1-2 mm.
Water in technology and beyond
For use in technology, water, mostly in a liquid state, sometimes also in the form of water vapour or ice, is very important.
- The weight of water is used in water mills and water turbines to generate mechanical or electrical energy.
- Steam is used in steam turbines, which are found in many modern power plants, to convert the primarily generated heat first into mechanical energy and finally into electrical energy with a generator. The (piston) steam engine was particularly important in the industrial revolution.
- Ice machines are used to produce and process ice cream and ice cubes. The ice processing machine enables the smoothing of artificial ice rinks.
- In heat transfer, water is used as a means of transport for heating or in water cooling. Water can also be used to generate cold by evaporation. Refrigeration machines work on the basis of the adsorption of ammonia in water or water vapour in (aqueous) lithium bromide solution.
- Water is used cold and hot for washing and cleaning, often in combination with detergents, alkalis or acids. It also enables dissolving (leaching of salt deposits), separating via chromatography or extracting (infusions), recrystallising (setting of gypsum or cement together with carbon dioxide). It can also be used in the form of a pressure jet for high-pressure cleaning or for water jet cutting, for example in hygiene-sensitive areas.
- In the form of gel, water is used as a sound transmission medium from the sensor head to the human body in ultrasound diagnostics. Water is a sound transmission medium in echo sounding.
- As a medium with high surface tension and a good evaporation rate, water is used for the sliding clapping of labelling foil on shop windows, car bodies and other smooth surfaces to be laminated, as well as a lubricant and sealant for suction cups. The surface tension of water, in combination with soap, allows soap bubbles and the construction of layers from molecular thickness and fine membranes for physical experiments. The water runner can run by means of dents in the surface, biofilms can spread.
- Original hydraulics use water as a pressure transmission medium as well as fountains in fountains and water features, which also provide evaporative cooling and lighting effects. Fracturing geological strata in fracking is also a high-pressure application.
- Buoyancy created by water allows creatures, ships and buoys to float. Ballast tanks help stabilise unloaded or unevenly loaded ships. For submarines, they enable them to ascend and descend. There are also applications in the field of cableways, when these lifts are pulled or lifted in return by water ballast tanks.
- Water as a dissociation medium is used in electrolysis, electroplating, accumulator and battery technology as well as in old power plants as a power control tank. Furthermore, water is used as a solvent in aqueous chemistry.
- In medicine, water is used as a solvent for injecting or infusing substances into the body to correct the body's water balance, for softening and for rinsing.
- Water makes it possible to bend willow rods, cane, etc. by soaking them in water. Hardwood is formed into bentwood furniture under steam.
- Water can filter out infrared radiation from light bulbs and absorbs ionising radiation in the decay basins of nuclear power plants.
- Water, with and without chemical additives, is used as ammunition in water cannons.
- Ultra-pure water conducts electricity poorly. Only when other substances that can dissolve into ions are added can it transmit electric current.
- In nuclear power plants, water is used as a moderator, i.e. to slow down neutrons.
Water treatment
Nitrogen compounds, heavy metals, salts and hydrocarbons that were already present in the surface water can often be detected in extracted raw water. Since these substances are harmful to health, they must be removed by various processes.
Afterwards, the water must be passed through special filters filled with activated carbon. Activated carbon can attach substances to very large surfaces and thus remove them from the water.
Only now can the water be called drinking water. Before it is fed into the supply network, it is disinfected, if necessary, to be on the safe side.
The Water Technology division of Donau Chemie AG develops, produces and sells precipitants and flocculants based on iron and aluminium chlorides for the wide-ranging treatment of water as well as products for special industrial applications. The product spectrum ranges from municipal and industrial wastewater treatment to municipal drinking and bathing water treatment to solutions for the paper industry and products for optimising biogas processes.
Water and climate change
According to a study by the EU Commission, droughts have increased in the European Union over the past 30 years. While this has mainly affected southern and south-eastern Europe so far, areas in central and eastern Europe will also suffer more from water shortages in the future. Here it is important to secure the water supply and to counteract the waste of water. The basis for this is the EU Water Framework Directive of 2000, which stipulates that the Member States must use water more efficiently by 2010.
Climate forecast for Austria
In Austria, too, water must be available to everyone in good quality, and wastage must be avoided as a matter of principle. Already now, the
effects of lack of rainfall and
droughts are clearly noticeable.
According to forecasts by climate researchers, the annual total of precipitation in Austria will remain the same, but the south will receive less of it than the area north of the main Alpine ridge. In winter, more precipitation will come in the form of rain and less in the form of snow, so that fewer groundwater reserves can be formed. The glaciers will continue to recede. The south, especially Carinthia and eastern Styria, will be almost entirely affected by a decrease in precipitation.
Conclusion: Water is not an inexhaustible resource - neither in Austria
As the basis of all life, water is a precious commodity that must be treated with care. By using water sparingly in both the private and industrial sectors, as well as through professional recycling, private individuals and companies can contribute to ensuring water quality for future generations.
A global solution is more necessary than ever due to the increasing population density as well as the industrial, commercial and agricultural development of the last decades.
For more detailed information on our products for the treatment and processing of drinking water, pool water and waste water, as well as odour & corrosion control, please contact us at any time.
 www.donauchem.at
Sources:
www.donauchem.at
Sources:
https://www.derstandard.at/story/2000137296077/seen-bei-wiener-neustadt-statt-wasser-haben-wir-stinkenden-heilschlamm (Stand: 9.3.2023)
https://kurier.at/wissen/wissenschaft/duerre-die-wasserversorgung-wird-selbst-in-oesterreich-zum-problem/402304493 (Stand 9.3.2023)
http://www.wasserwerk.at/home/alles-ueber-wasser (Stand 9.3.2023)
https://klassewasser.de/content/language1/html/3697.php (Stand 9.3.2023)
http://wasseraktien.de/wasser/wasser-als-lebensgrundlage (Stand 9.3.2023)
https://www.umweltbundesamt.de/daten/wasser (Stand 9.3.2023)