Activated carbon has established itself as an extremely effective solution for air and gas purification. The use of activated carbon obtained from coconut shells deserves special attention. Due to its outstanding properties, this variant is proving to be a promising alternative to coal-based activated carbons in certain applications. We take a look at the specific advantages of coconut activated carbons and their versatile applications in gas and air purification.
We compare Alcarbon® UC 50/4x8 (coconut carbon) and Desorex® K 43 (hard coal) as representative grades.
Advantages of activated coconut carbon compared to hard coal
Activated carbon has become indispensable in the chemical industry. Its use in gas and air purification ranges from the recovery of valuable raw materials to the desulphurization of biogas and the removal of organic compounds. In addition, growing environmental awareness and strict emission limits are opening up new areas of application, particularly in the removal of pollutants from exhaust air.
Choosing the right activated carbon is not always easy. However, for some applications in gaseous media, coconut carbon has clear performance advantages over hard coal, which can be summarized as follows:
1. Higher micropore content
- Advantage: The amount of a substance that can be adsorbed depends to a large extent on the size of the inner surface of an activated carbon. The higher proportion of micropores in coconut shell-based activated carbon provides a larger inner surface area and, above all, increases the adsorption capacity for smaller and more volatile pollutants.
- Application: Higher adsorption capacity in the presence of small quantities of pollutants and volatile substances (VOCs).
2. Higher hardness
- Advantage: The natural hardness of the coconut shells gives the activated carbon a resistant structure that better withstands the stresses in certain gas and air purification applications.
- Application: Higher resistance and lower carbon shrinkage against aggressive media.
3. Lower ash content
- Advantage: A low ash content plays a decisive role in applications in which organic compounds react strongly exothermically with the ash content of the activated carbon. The use of activated carbon with a low ash content, as found in coconut carbon, minimizes potential fire risks.
- Application: Reduces the risk of fire in the presence of ketones.
4. Renewable raw material
- Advantage: The use of coconut shells as a raw material for activated carbon is not only efficient, but also sustainable. The use of a renewable raw material helps to minimize environmental impact and reduce the ecological footprint.
- Application: Improving the CO2 balance.
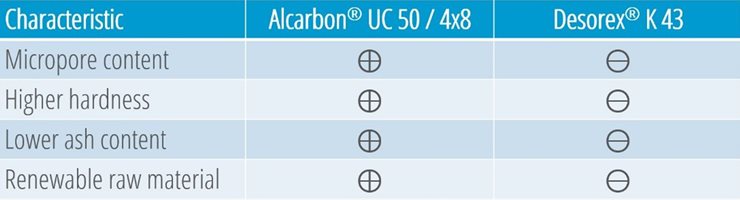
Illustration 1: Comparison of Alcarbon® UC 50/4x8 (coconut carbon) and Desorex® K 43 (hard coal)
Adsorption on coconut activated carbon and hard coal
The following comparative study of the adsorption isotherms of
Alcarbon® UC 50/4x8 (coconut carbon) and
Desorex® K 43 (hard coal) provides insight into the performance of coconut shell-based activated carbon in the adsorption of certain substances from a surrounding gas phase.
For a better understanding of the adsorption isotherms, here are some definitions:
1. Adsorption:
- Definition: Adsorption describes the accumulation of a gaseous substance (= adsorptive) on the inner surface of the activated carbon (= adsorbent).
- Example: When activated carbon comes into contact with a gaseous medium, such as air, which contains volatile organic compounds (VOCs), this leads to the activated carbon being loaded with these VOCs, which reduces the concentration of VOCs in the air.
2. Adsorption capacity:
- Definition: The adsorption capacity is the amount of a substance to be adsorbed that can be absorbed by a certain amount of activated carbon.
- Example: If the adsorption capacity for the substance benzene is 20%, this means that the activated carbon can adsorb up to 20% of its weight in benzene before it is considered saturated. In other words, 1 kg of activated carbon can adsorb 200 g of benzene.
3. Adsorption isotherm/equilibrium:
- Definition: The adsorption isotherm is the graphical representation of the adsorption capacity as a function of the concentration of the adsorptive.
- Example: An adsorption isotherm for benzene shows how the adsorption capacity of the activated carbon increases with increasing benzene concentration in the air until a state of equilibrium is reached.
The adsorption isotherms of the following examples show the course of the equilibrium loading as a function of the concentration:
1. Loading (%):
- Definition: The equilibrium loading is the adsorbable quantity of a substance at a certain concentration, based on the total weight of the activated carbon, expressed as a percentage, above which no further loading takes place, as the proportion of adsorption corresponds to that of desorption.
- Example: If the equilibrium loading for benzene is 15%, this means that 15% of the weight of the activated carbon can be adsorbed on benzene until a state of equilibrium is reached between the uptake (adsorption) and release (desorption) of benzene.
2. Concentration (g/m3):
- Definition: The concentration corresponds to the mass of the substance to be adsorbed per volume of the surrounding medium, measured in grams per cubic meter.
- Example: A concentration of 5 g/m³ benzene in air means that 5 grams of benzene are present in every cubic meter of air.
Note: The adsorption capacity or equilibrium loading of an activated carbon for a specific substance is always concentration-dependent. The amount that can be adsorbed until the equilibrium state between adsorption and desorption is reached increases with increasing concentration. This is important to understand the performance and efficiency of adsorption processes.
Coconut activated carbon for low levels of pollutants
In numerous exhaust air and odor treatments, predominantly low pollutant concentrations occur, such as in industrial parks near residential areas. Regardless of the specific pollutant substance, activated carbons with a high proportion of micropores are better suited to adsorbing low levels of pollutants than open-pored activated carbons. The microporous structure offers a larger surface area and therefore a more effective interaction with the small quantities of pollutants in the exhaust air.
Example: Benzene (C₆H₆)
Benzene is a volatile organic compound (VOC) that evaporates into the air at room temperature.
 Illustration 2: Comparative study of the adsorption isotherms of Alcarbon® UC 50/4x8 (coconut carbon) and Desorex® K 43 (hard coal) with benzene at 20 °C.
Illustration 2: Comparative study of the adsorption isotherms of Alcarbon® UC 50/4x8 (coconut carbon) and Desorex® K 43 (hard coal) with benzene at 20 °C.
Coconut activated carbon for volatile compounds
Small molecules with a low molecular weight are difficult to adsorb because they have certain properties:
- Volatility/volatility: These molecules are very volatile, which indicates that they can evaporate strongly at comparatively low temperatures.
- Low boiling points: They have low boiling points, which means that they change to the gaseous state even at low temperatures.
- High vapor pressures: Due to their volatility and low boiling points, these molecules have high vapor pressures.
Coconut shell-based activated carbons with a high proportion of micropores are particularly suitable for the adsorption of such volatile compounds. Typical examples of molecules of this type that are difficult to adsorb are
- organic compounds containing sulphur,
- halogenated hydrocarbons and
- short-chain hydrocarbons.
1. Sulfur-containing organic compounds
Example 1: Carbon disulphide (CS₂)
Carbon disulphide, also known as carbon disulphide, is a volatile organic sulphur compound that is found in biogas and is formed during decomposition processes.
 Illustration 3: Comparative study of the adsorption isotherms of Alcarbon® UC 50/4x8 (coconut carbon) and Desorex® K 43 (hard coal) with carbon disulphide at 20 °C.
Illustration 3: Comparative study of the adsorption isotherms of Alcarbon® UC 50/4x8 (coconut carbon) and Desorex® K 43 (hard coal) with carbon disulphide at 20 °C.
Example 2: Dimethyl sulfide (C₂H₆S)
Dimethyl sulphide (DMS) has a very low boiling point of 37 °C and mainly occurs in biogas. One problem with this highly volatile compound is that it can only be adsorbed physisorptively. This means that, despite the low boiling point, adsorption is not based on the chemical bond between adsorbate and adsorbent (chemisorption), but on physical interactions, the Van der Waals forces. The micropores in the coconut carbon provide an ideal environment for the physisorptive adsorption of DMS.
 Illustration 4: Comparative study of the adsorption isotherms of Alcarbon® UC 50/4x8 (coconut carbon) and Desorex® K 43 (hard coal) with dimethyl sulfide at 20 °C
Illustration 4: Comparative study of the adsorption isotherms of Alcarbon® UC 50/4x8 (coconut carbon) and Desorex® K 43 (hard coal) with dimethyl sulfide at 20 °C
2. Halogenated hydrocarbons
Example 1: Dichloromethane (CH2Cl2)
Dichloromethane (DCM), also known by the trivial name methylene chloride, is a colorless liquid that is difficult to ignite. With a boiling point of 39.8 °C, dichloromethane is a highly volatile substance. Due to these properties, it is often used as a solvent for various applications, including resins, greases, plastics and bitumen. Here too, coconut shell-based activated carbon shows its superiority over hard coal due to its microporous structure.
 Illustration 5: Comparative study of the adsorption isotherms of Alcarbon® UC 50/4x8 (coconut carbon) and Desorex® K 43 (hard coal) with dichloromethane at 20 °C.
Illustration 5: Comparative study of the adsorption isotherms of Alcarbon® UC 50/4x8 (coconut carbon) and Desorex® K 43 (hard coal) with dichloromethane at 20 °C.
Example 2: Trichloromethane (CHCl3)
Trichloromethane, known by the common name chloroform, is a colorless, non-flammable, volatile liquid with a sweet odor. Chloroform is primarily used as a solvent and for the production of chlorofluorocarbons (CFCs). At normal pressure, the boiling point is 61 °C. Due to its volatile nature, chloroform also evaporates strongly at lower temperatures. The coconut shell-based activated carbon with its microporous structure proves to be particularly effective in adsorbing chloroform from gaseous media.
 Illustration 6: Comparative study of the adsorption isotherms of Alcarbon® UC 50/4x8 (coconut carbon) and Desorex® K 43 (hard coal) with chloroform at 20 °C.
Illustration 6: Comparative study of the adsorption isotherms of Alcarbon® UC 50/4x8 (coconut carbon) and Desorex® K 43 (hard coal) with chloroform at 20 °C.
3. Short-chain hydrocarbons
Short-chain hydrocarbons are a group of hydrocarbons whose molecules have lower molecular weights than their long-chain counterparts. The following applies:
The shorter the chain, the higher the hydrocarbon,
- the lower its molecular weight,
- the lower its boiling point,
- the higher its vapor pressure,
- the more difficult it is to adsorb.
An exemplary scenario of these relationships can be illustrated using alkanes (kerosenes): pentane with five carbon atoms has a lower tendency to adsorb than hexane with six carbon atoms, etc. (pentane < hexane < heptane < octane < ...).
Coconut activated carbon with its above-average proportion of micropores has proven its worth in achieving optimized interaction and adsorption of small molecules, such as those present in short-chain hydrocarbons.
Example: Pentane (C5H12)
 Illustration 7: Comparative study of the adsorption isotherms of Alcarbon® UC 50/4x8 (coconut carbon) and Desorex® K 43 (hard coal) with pentane at 20 °C.
Illustration 7: Comparative study of the adsorption isotherms of Alcarbon® UC 50/4x8 (coconut carbon) and Desorex® K 43 (hard coal) with pentane at 20 °C.
Coconut activated carbon in the presence of ketones
Adsorption is generally an exothermic process, i.e. heat of adsorption is released. Ketones contain a non-terminal carbonyl group (C=O) as a functional group, which is chemically reactive and also reacts strongly exothermically with the ash content of the activated carbon. There is a higher risk of heat development and potentially also a fire hazard (formation of "hot spots"). Therefore, in the presence of ketones, activated carbon with a low ash content, such as coconut carbon, is important.
Prominent representatives of ketones are propanone (acetone), butanone (methyl ethyl ketone, MEK), methyl isobutyl ketone (MIBK) and cyclohexanone (azocyclohexane).
Example 1: Propanone (C3H6O)
Propanone, better known as acetone, is a polar aprotic solvent with many applications in the chemical industry. Due to its properties, acetone is often used as a starting material for numerous syntheses in organic chemistry.
 Illustration 8: Comparative study of the adsorption isotherms of Alcarbon® UC 50/4x8 (coconut carbon) and Desorex® K 43 (hard coal) with acetone at 20 °C.
Illustration 8: Comparative study of the adsorption isotherms of Alcarbon® UC 50/4x8 (coconut carbon) and Desorex® K 43 (hard coal) with acetone at 20 °C.
Example 2: Butanone (C4H8O)
Butanone, also known by the common name methyl ethyl ketone (MEK), is one of the most important industrially used ketones alongside acetone. It is mainly used as a solvent in various industrial applications.
 Illustration 9: Comparative study of the adsorption isotherms of Alcarbon® UC 50/4x8 (coconut carbon) and Desorex® K 43 (hard coal) with 2-butanone at 20 °C.
Illustration 9: Comparative study of the adsorption isotherms of Alcarbon® UC 50/4x8 (coconut carbon) and Desorex® K 43 (hard coal) with 2-butanone at 20 °C.
Coconut activated carbon for aggressive media
Coconut activated carbon is highly resistant to a wide range of aggressive chemicals. The robust structure extends its life and ensures stable performance, even under demanding conditions. These properties make it a preferred solution in industrial processes where a high hardness activated carbon is required.
Example 1: Catalytic decomposition
Under oxidation of the activated carbon surface, some substances are not adsorbed but catalytically decomposed. In contrast to pure adsorption, in which molecules are bound to the inner surface of the activated carbon, catalytic decomposition leads to the chemical conversion of these molecules on the outer surface. A characteristic feature of this process is the carbon shrinkage or loss that occurs during catalytic decomposition. This loss decreases with the hardness of the activated carbon, which is why activated carbons with high hardness are preferred.
A concrete example of catalytic decomposition on activated carbon is the decomposition of ozone molecules. This shows that the hardness of coconut carbon creates an optimal environment that efficiently enables the catalytic decomposition of ozone and other pollutants.
Comparison of the CO2 balances of hard coal and coconut activated carbon
Companies are increasingly focusing on the carbon footprint of products. A comparison of the CO2 balances clearly shows that activated carbon made from coconut shells performs significantly better than hard coal products: The production of coconut carbon generates only a fraction of CO2 equivalents compared to hard coal. If you consider the entire life cycle of activated carbon, including reactivation and disposal, the use of coconut carbon is even more environmentally friendly.
You can read how Donau Carbon calculates the CO2 footprint of activated carbon in our article "Activated carbon and CO2 footprint"
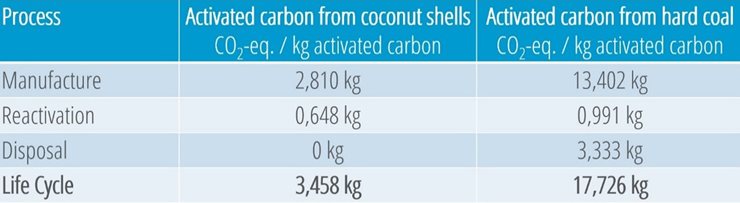
Illustration 10: Comparison of the CO2 balances of activated carbon from coconut shells and hard coal. © Donau Carbon
Conclusion: Coconut activated carbon in gas and air purification
The use of activated carbon has established itself as an extremely effective solution for air and gas purification. In certain applications, coconut activated carbon shows clear performance advantages over coal-based variants.
The higher micropore content in coconut shell-based activated carbon provides a larger internal surface area and increases the adsorption capacity for difficult-to-adsorb pollutants, especially low levels and volatile substances. The low ash content minimizes the risk of fire in the presence of ketones, while the natural hardness of coconut activated carbon ensures greater resistance to aggressive media. The comparison of the CO2 balances also underlines the environmental friendliness of coconut carbon compared to hard coal.