C for Cellulose
Cellulose is the main component of plant cell walls and thus the most common organic compound in nature. It is also an important store for the element carbon and thus essential for the carbon cycle.
Cellulose is a polysaccharide (multiple sugar), which means it consists of several hundred to ten thousand glucose molecules, which makes it a so-called macromolecule. This is formed by the reaction (polycondensation) of many glucose molecules with the splitting off of water.
Applications in everyday life
Humans have been using the natural substance cellulose for a very long time. It is usually obtained from wood or straw, but cotton, hemp, jute and flax also consist almost entirely of the fibrous material. The wood of deciduous trees and conifers consists of about 40% cellulose. The stalks of cereal plants (straw) are also rich in cellulose, and the seed hairs of cotton consist almost entirely of cellulose. Cellulose is thus the most important natural substance in terms of quantity and forms an essential scaffolding and supporting substance.
Diverse use in various industries
Wood has always been valuable to man as a building material. Cellulose has also gained more and more technical and economic importance, which is due to the multitude of its manifestations, that also characterise its multifaceted properties. Cellulose is used in the textile, paper and construction industries, but chemically modified cellulose derivatives are also becoming increasingly important for the food industry, e.g. as thickeners, fibre additives and fillers.
Depending on the type of use, there are several possibilities for technical extraction:
- the cellulose obtained from wood serves as a basic material in many industries of the paper industry.

- In the clothing industry, cellulose is used as regenerated cellulose fibre (viscose), cotton fibre and linen.

- Methyl cellulose/cellulose ether is a cellulose derivative used mainly in the building materials industry.

- Cellulose is also known as a basic material for the plastic known as cellophane (packaging industry, transparent cigarette paper).

- for the production of table tennis balls

- for the production of bioethanol
Cellulose ethanol
Cellulose ethanol is obtained through the bioconversion of cellulose and can be produced from plant residues and waste materials, making it one of the second-generation biofuels. Great hopes for the future are being placed in its research and development.
Until now, the use of plant cellulose has been difficult to hardly possible and bioethanol was mainly produced by the alcoholic fermentation of sugar in the form of starch (maize, grain) or sucrose (sugar beet, sugar cane). Since cellulose is a multiple sugar as well as the main component of plant cell walls and has a mass share of 50 percent, there is enormous potential for use in the energy sector. In addition, residual and waste materials can be used that do not compete with food and achieve much higher CO2 savings.
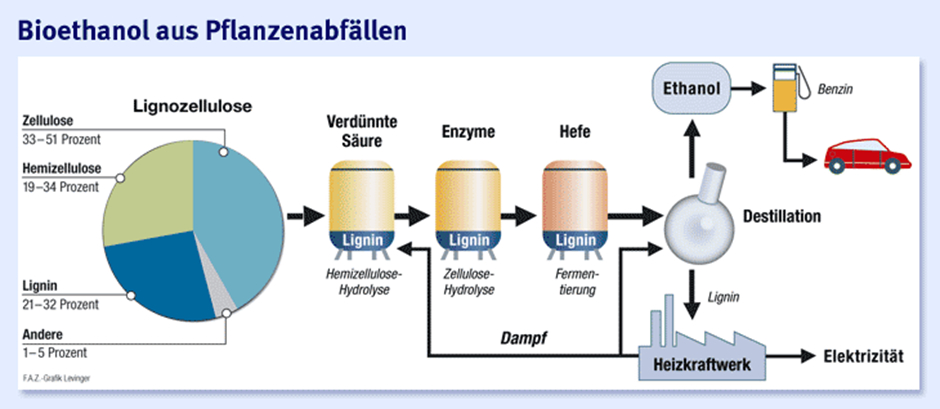
Source: www.faz.net
Cellulose as a carbon dioxide store
Photosynthesis removes carbon dioxide from the atmosphere. The carbon contained in organic matter is largely bound in cellulose. The carbon stored in the form of cellulose accounts for about 50% of the carbon present in the entire earth's atmosphere as CO2.
Cellulose in food
Cellulose is called dietary fibre because the human body has no digestive enzymes for breaking down cellulose. Together with hemicelluloses (short-chain cellulose), pectin and lignin, it forms the main part of dietary fibre in foods of plant origin. Thanks to anaerobic bacteria in the mucous membrane of the large intestine, however, cellulose can be metabolised to short-chain fatty acids, which we humans can then utilise.
Ruminants, on the other hand, can digest cellulose and other polysaccharides in the rumen as the bonds are broken down here by the rumen microorganisms. The same applies to horses and fowl. In these animals, microbial fermentation takes place in the large intestine. Certain fungi and the silverfish are also able to digest cellulose.
Food additive
Cellulose is used in the food and pharmaceutical industries, e.g. in tablets as a building material. As a food additive, it bears the designations E 460 to E 466.
- E 460i - Microcrystalline cellulose
- E 460ii - Cellulose powder
- E 461 - Methyl cellulose
- E 463 - hydroxypropyl cellulose
- E 464 - Hydroxypropylmethylcellulose
- E 465 - ethyl methyl cellulose
- E 466 - carboxymethyl cellulose
Chemical properties of cellulose
Cellulose is insoluble in water and in most organic solvents and hardly swells. However, it can be split by strong acids and can also be degraded to glucose at elevated temperatures with the help of concentrated acids.
With the molecular formula C12H20O10, its structural formula is conspicuous because the oxygen of the glycosidic bond between the molecular building blocks points alternately upwards and downwards. This is because the direction (up or down perpendicular to the "ring plane") in which the glycosidic OH group points at the monosaccharide building block is not variable, and points in the opposite direction as the OH group at the C-4 atom.
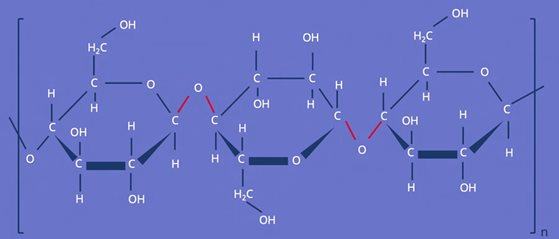
Source: studyflix.de
 www.donauchem.at
Related Links:
www.lernhelfer.de
www.chemie.de
studyflix.de
www.spektrum.de
www.cleanenergy-project.de
www.donauchem.at
Related Links:
www.lernhelfer.de
www.chemie.de
studyflix.de
www.spektrum.de
www.cleanenergy-project.de